By John B. Marler
Organic nitrogen and inorganic nitrogen: what’s the difference?
Organic growers frequently attempt to quantify the amount of organic nitrogen they add to their soil ecosystems in the same manner that conventional growers use inorganic nitrogen units to calculate their nitrogen requirements. Logically, they reason that a ton of organic material with 4 percent nitrogen content as verified by a laboratory test will provide 80 pounds, or units by some determinations, of nitrogen.
The truth is that organic nitrogen sources vary in their efficiency of transformation into soil components over a much broader range of response than do inorganic synthetics, which offer precision measurement and a repeatable predictability of release. Use of inorganic nitrogen units to determine nitrogen needs for organic growers is therefore problematic. A popularly available and reliable conversion algorithm between tested inorganic nitrogen and untested organic nitrogen in organic soils does not exist, however. Without such an algorithm there can be no scientific basis of comparison.

A farmer gives a plant organic humus fertilizer to plant.
Synthetic nitrogen
Synthetic, inorganic nitrogen sources are of a totally different nature than organic nitrogen. The term organic in this context should not be confused with its broader usage, as in “organic growing.”
Here, it refers to the carbon nature of its molecular structure. Inorganic nitrogen, found in nitrite, nitrate, and ammonium forms, does not have carbon in its molecule.
Synthetic, inorganic nitrogen is usually found dissolved or in a readily water soluble form. Due to the unstable nature of synthetic nitrogen, it easily volatilizes into the atmosphere or is lost in ground or surface water.
University and USDA studies have revealed that the nutrients from 50 to 82 percent of all inorganic synthetic nitrogen fertilizers are lost into the atmosphere or into surface or ground water. This alone would indicate that inorganic nitrogen unit rates are inflated to allow for the inefficiency of use.
If this is true, then out of 100 synthetic nitrogen units, only 50 — or perhaps as little as 18 — are actually used by plants. An organic farmer might reasonably calculate his nitrogen needs with the understanding that his plants need 50 percent less nitrogen than inorganic tables would indicate.
Organic Nitrogen
Organic nitrogen, on the other hand, is much more efficient in providing nitrogen nutrition than labile, inorganic sources. The efficiency of organic sources is illustrated by applications on turf on sand-based golf courses. The inorganic nitrogen nutrients, which may range from 8 to 16 percent inorganic nitrogen, must be applied every 30 to 45 days to maintain an acceptable level of dark-green color.
By contrast, only two applications of 4-4-4 organic fertilizers per year, in spring and fall, will maintain a deeper and healthier green. In our research at the University of Florida, 100 percent organic 4-4-4 fertilizer outperformed 8-5-5 fertilizer that contained both organic and inorganic nitrogen.
What are the levels of efficiency of organic nitrogen in plant growth when compared to inorganic nitrogen? We are not sure. However, we can again turn to the turf industry for comparisons. We do know that more intensively groomed golf courses typically use four to eight applications of inorganic nitrogen per year, compared to two applications a year for low-nitrogen-level organic nitrogen.
That would indicate a 200 to 400 percent greater level of effectiveness for high-efficiency organic fertilizers.
We are aware of one golf course that attempted to apply organic nitrogen sources at the same rate as inorganic fertilizer. The result was that the course developed such a thick turf that the grass could not be mowed.
We also have repeatedly seen, in hundreds of reported applications, acceleration of growth in field crops, orchards and vineyards that would indicate that efficient low-nitrogen-content organic fertilizers can deliver adequate nitrogen to grow a superior crop and still leave high levels of measurable inorganic nitrogen in the soil after the crop is harvested. This was illustrated by inorganic testing by a major California grower that indicated that even after a broccoli crop — known for its high nitrogen demands — was harvested, there was almost enough inorganic nitrogen in the soil to grow a second crop.
The Nitrogen Cycle theory
Compounding the problem of attempting to measure organic nitrogen in inorganic units is the fact that plants seem to be more inclined to use organic nitrogen than inorganic. In spite of “known facts,” researchers, after an intensive study in remote, pollution-free forests, have recently determined that the major source of nitrogen in a pristine area is actually organic nitrogen.
For soil science this was an earthshaking revelation. The “fact” that inorganic nitrogen is the only plant-usable form has been taught in our universities for at least a century.
Early soil scientists drew samples of what they thought was soil-leaked nitrogen from streams and lakes near urban areas. These sources were actually polluted by human consumption of hydrocarbon-based fuels, including coal and wood.
In North American polluted surface waters, only about 2 percent of leaked nitrogen was determined to be organic, while about 70 percent of the tested nitrogen was inorganic in nature. North American field testing supported European science that was seen to confirm the theory that plants can only use inorganic nitrogen. Slowly, that flawed field science developed into what we now know as the “Nitrogen Cycle.”
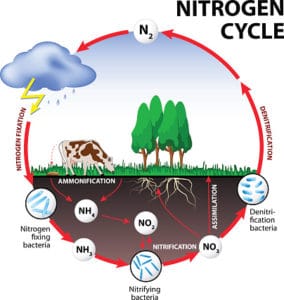
That longstanding theory was called into question, however, when very low levels of inorganic nitrogen, about 5 percent, were found in water sources in pristine areas. This new research suggests that plants have to efficiently use organic nitrogen — otherwise those pristine forests would not exist. Those of us who were taught the “Nitrogen Cycle” are now faced with learning new principles and revisiting our concepts of what forms of nutrition plants can use.
From our own research, and the research of others, we believe that the soil functions by building small sources of nitrogen, both inorganic and organic, using a wide range of mechanisms. Most of these mechanisms are microbiological factors that don’t seem to add up to much until measured together as a whole.
An example is the transformation of bacteria into nitrogen. Within a short time after the application of an organic food source that meets a bacterium’s needs, there is a massive explosion of the population of bacteria, followed shortly by an equally massive explosion of a population of beneficial nematodes that feed on the bacteria. Some microbiologists, such as Elaine and Russ Ingham, have suggested that a soil count of these nematodes offers a good means of determining the value of soil nutrition.
With a short life cycle — measured in days or maybe a week or two — beneficial nematodes will bloom within a soil in response to the increase in their food source. As they live, reproduce, and die, they leave behind elevated levels of nitrogen (most microbes have about 17 percent nitrogen content in their bodies). The result of these short life cycles is an increase in both organic and inorganic nitrogen.
This mechanism, along with many others that naturally add small incremental additions of nitrogen and other soil nutrients, occurs within organic soils only when the soils get the proper nutrition. Availability and application of soil minerals, including some aspects of the fixing of organic nitrogen in the soil, are often conditioned upon the presence of other soil minerals.
The Nitrogen Dilemma
An organic grower armed with this new information is faced with a dilemma. Some agronomists will no doubt continue to argue that the system of testing and application for synthetic, inorganic nitrogen is still applicable in conventional growing programs. That argument has real merit, given that their current science is based on the testing of inorganic nitrogen.
Arguing the validity of using synthetic inorganic testing and measurement programs for organic growing is another matter. If a grower is working to build up organic soils, which rely on organic nitrogen, then why test and measure inorganic nitrogen? There is no doubt that there is a correlation between inorganic levels and organic levels. Inorganic nitrogen transformations from organic nitrogen occur continually. Inorganic nitrogen is present in all organic soils. However, the primary standard toward which organic growers should work is the establishment of long-term, slow and steady organic nitrogen sources, not transient inorganic nitrogen.
Instead of focusing on short-term inorganic nitrogen sources like their conventional growing cousins, organic growers should concentrate on building up organic nitrogen sources in the form of soil acid gels in their soils. Any source of organic matter will, sooner or later, become a soil acid after it has deteriorated into the soil. As the soil acid absorbs moisture it becomes a soil acid gel. However, not all soil acids are equal in nature. Soil acids are widely varied as to the complexity of their molecular structure and their value to plants. The chelated (elements with carbon bonds, usually in the form of amino acids) nutrients that are available to the soil acid at the time of formation will become an immediate part of its molecular structure. For example, if chelated nitrogen and copper are available, then nitrogen and copper will become part of the soil acid molecular structure. The more complex molecular structures are the result of complex organic nutrition sources available at the time of molecule construction.
In the formation of organic nutrients, the old adage of “garbage in, garbage out” holds true. Higher-quality organic forms are much more efficient at transformation into soil acids with more complex nutrient structures. A ton of green leaves obviously has more overall organic nutritional value than a ton of dried, partially deteriorated leaves. A ton of “hot,” or nutritionally loaded, fresh chicken manure obviously has more nutrients than a ton of “cold” or aged chicken manure.
That said, the hot manure may actually be a less efficient transformer than the cold manure due to its labile nature. While a snapshot, in the form of a laboratory test, of the inorganic nitrogen content is often possible, that test can quickly be rendered invalid by a hot drying wind or hindered by a cold snap that slows or stops nutrient transformation from the soil into soil acids. Bacteria content and the nature of the bacteria, pH, the chelated nature of the material, carbon content (such as animal bedding materials) presence of animal treatment materials (such as arsenic and antibiotics) size of the material, and internal temperature are all factors that can affect the amount of nutrients available for use by plants. Ambient temperature, sunlight, and wind are also large factors. Manure that is surface applied without soil cover can lose 25 percent of its nutrients in a single 24-hour period on a sunny, windy day.
The efficiency of transformation, which is a measurement of how efficient a decomposing organic is transformed into valued complex soil acid gels, is the single most important aspect involved in adding organic materials to a soil. Organic growers should focus on the efficiency of transformation of all organic nutrient supplements they add to their soils. Mineral and nutrient rich organic material that is slow release is of much greater value than that of a labile nutrient that volatilizes into the air and water around it. We have been in fields thick with the smell of releasing ammonia. Such a smell indicates a failure of the grower to capture the full nutrients from the organic material he has applied. Typically, the efficiency of transformation in a field that has the distinct smell of manure is low. High efficiency organic fertilizers have little or no smell after application.
While organic growers may benefit from knowing the levels of inorganic nitrogen in the soil, this measurement alone is almost insignificant when compared to the question of how much organic nitrogen is in the soil. The only reasonable means of measurement at this time is inference based on fulvic and humic acid levels in the soil. These tests are significant since they reflect the true nature of soil carbon and are reliable indicators of available soil nutrients.
Editor’s Note: This article was originally published in the July 2006 issue of Acres U.S.A.

